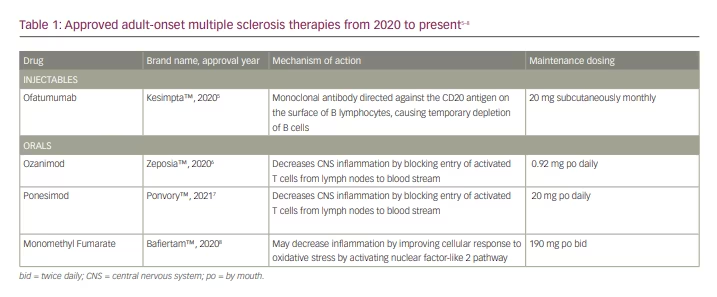
Paediatric-onset multiple sclerosis (POMS) is the symptom onset and diagnosis of multiple sclerosis (MS) before the age of 18.1 Diagnostic criteria were revised by the International Pediatric MS Study Group in 2013. Compared to patients with adult-onset MS (AOMS), young patients are less likely to develop primary or secondary progressive MS and more likely to follow a relapsing–remitting course. Although early relapses in patients with POMS tend to be more highly inflammatory and more frequent,2 children seem to demonstrate more resiliency, recovering more quickly and effectively from each attack. However, there is growing evidence that patients with POMS still require early treatment with a highly effective disease-modifying therapy (DMT) to help prevent significant long-term disability.3,4 Over the past decade, there has been a rapid development of new DMTs for AOMS, including four that have been newly US Food and Drug Administration (FDA)-approved in the past 2 years (Table 1).5–8 These include ofatumumab and several new S1P receptor modulators (Table 1). Ofatumumab is a B-cell depleting therapy similar to rituximab and ocrelizumab but is given by monthly subcutaneous injection following an induction of three weekly injections.5 After observation of the first dose to ensure safety, patients can then dose themselves at home without the need to travel to an infusion centre. The new S1P modulators were developed as alternatives to fingolimod, with the goal of improved safety such as a lack of stringent first-dose cardiac monitoring with some of the new products.9 However, the short- and long-term safety, dosing and effectiveness of most AOMS DMTs in the POMS population requires further clarification. New clinical trials exploring DMT use in POMS continue to emerge.
From 2006 through 2020, a total of three treatment trials analysing DMT use in POMS have been published. These include interferon β-1a, fingolimod and teriflunomide.10–12 Interferon β-1a is a first-generation injectable interferon requiring frequent dosing, fingolimod is a once-a-day oral sphingosine 1-phosphate (S1P) receptor modulator and teriflunomide is a once-a-day oral pyrimidine synthesis inhibitor.
The first treatment trial examined interferon β-1a use in 16 children with POMS, demonstrating overall stable median Expanded Disability Status Score and magnetic resonance imaging (MRI) activity in three patients, with worsening in six patients. It was well-tolerated, with side effects including flu-like symptoms, isolated myalgias and injection-site reactions. Treatment was not interrupted in any cases due to adverse events.10 Compared with interferon β-1a, the fingolimod trial demonstrated a relative decrease of 88% in adjusted annualized relapse rate, 53% reduction in annualized rate of new or newly enlarged T2 lesions and a higher rate of serious adverse events.11 A 2-year follow-up analysis showed sustained reduction in MRI activity and annualized rate of brain atrophy,13 continued control of disease activity and less disability progression.14 The third treatment trial compared teriflunomide against placebo in 166 patients with POMS, demonstrating no significant difference in time to first confirmed clinical relapse between groups, and a reduction in MRI lesions in the teriflunomide group compared with placebo.12 The safety profile was similar as that observed in adult patients, although there was a higher rate of pancreatitis with teriflunomide in paediatric patients compared to adults. This has only been reported in adult patients post-marketing.12,15
Most POMS DMT data comes from two retrospective analysis papers published in 2018 and 2020.16,17 For both analyses, clinical data was collected prospectively from a network of 12 Pediatric MS Centers throughout the USA and Canada. The 2020 retrospective paper looked at a total of 741 paediatric patients with MS and clinically isolated syndrome between 2011 and 2019 using propensity scores.17 The most significant finding was that early initiation of a highly effective DMT (as defined in the adult literature) led to more favourable outcomes compared to injectables.18 Furthermore, the DMT safety profiles were overall similar to those observed in adult trials.16,17
The purpose of this review is to provide updates on the most recent research investigating DMT use in POMS.
Methods
This narrative review includes a study of both publications and clinical trials published after 2020. For recent POMS DMT trials, a search was conducted using ClinicalTrials.gov by entering “pediatric multiple sclerosis” under [Condition or disease]. The following filters were subsequently checked under [Recruitment Status]: “recruiting”, “active not recruiting” and “completed”. To restrict the results to only recent trials, all “completed” studies prior to 2020 were excluded. All “active” studies, even those prior to 2020, were included, as some of the follow-up analyses have not yet been completed. Additional filters applied included “child (birth-17)” for [age] and “interventional” for [study type]. To restrict the results to include only drug treatment trials, only those listing the name of a specific MS DMT listed under the “intervention” column were included.
For recent publications, a search was conducted using PubMed by entering the following keywords under [Title/Abstract]: “pediatric multiple sclerosis” and the names of all available MS DMTs. To restrict the results to include only the most recent articles, date range filters were set to between “2020/01/01” and “2021/12/31”. All article types were subsequently included in the results.
Results
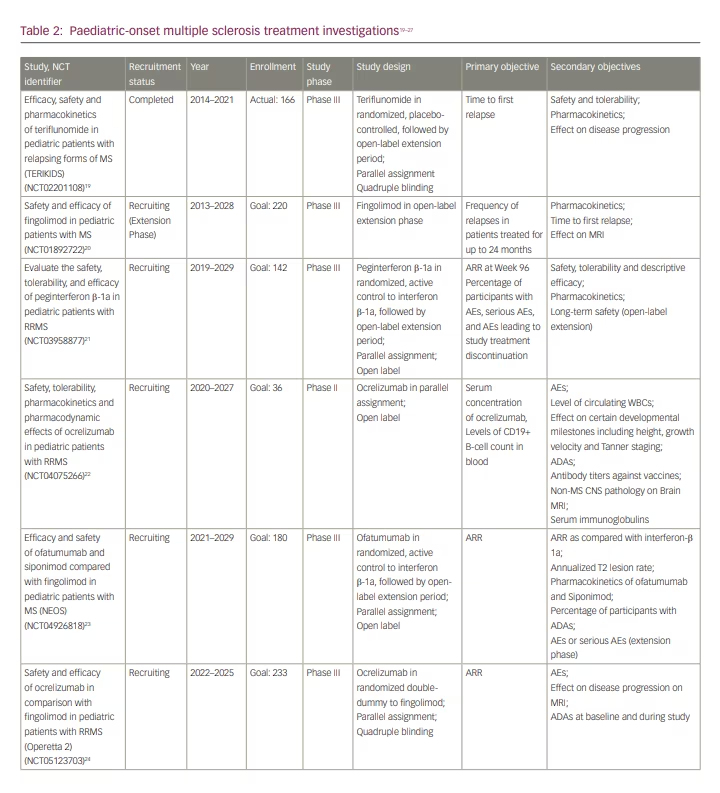
The POMS clinical treatment trials search yielded nine investigations (Table 2)19–27 and the publications search yielded 10 publications (Table 3).12,13,28–35 Of the nine new clinical treatment trials, there were four phase III investigations initiated after 2019 (Table 2). The first, comparing dimethyl fumarate (DMF) with peginterferon β-1a, aims to show that DMF is effective and safe enough to warrant its use as a first-line therapy over first-generation injectables (Study to evaluate the efficacy and safety of dimethyl fumarate [Tecfidera] and peginterferon beta-1a [Plegridy] for the treatment of relapsing-remitting multiple sclerosis in pediatric participants; ClinicalTrials.gov identifier: NCT03870763).27 The second, comparing ocrelizumab with fingolimod, was likely designed for two major reasons: to demonstrate that ocrelizumab is at least as effective as fingolimod and therefore warrants FDA approval in POMS, and to investigate whether ocrelizumab is more effective than fingolimod and thus should serve as the preferred first-line option for patients with POMS (A study to evaluate safety and efficacy of ocrelizumab in comparison with fingolimod in children and adolescents with relapsing-remitting multiple sclerosis [Operetta 2]; ClinicalTrials.gov identifier: NCT05123703).24 The third trial, which compares ofatumumab and siponimod to fingolimod, also likely aims to clarify the optimal first-line DMT in POMS (Efficacy and safety of ofatumumab and siponimod compared to fingolimod in pediatric patients with multiple sclerosis [NEOS]; ClinicalTrials.gov identifier: NCT04926818).23 Siponimod is a newer once-a-day oral S1P modulator alternative to fingolimod.9 The fourth trial compares peginterferon β-1a with the active comparator interferon β-1a to evaluate its effectiveness and safety in POMS (A study to evaluate the safety, tolerability, and efficacy of BIIB017 [peginterferon beta-1a] in pediatric participants for the treatment of relapsing-remitting multiple sclerosis; ClinicalTrials.gov identifier: NCT03958877).21
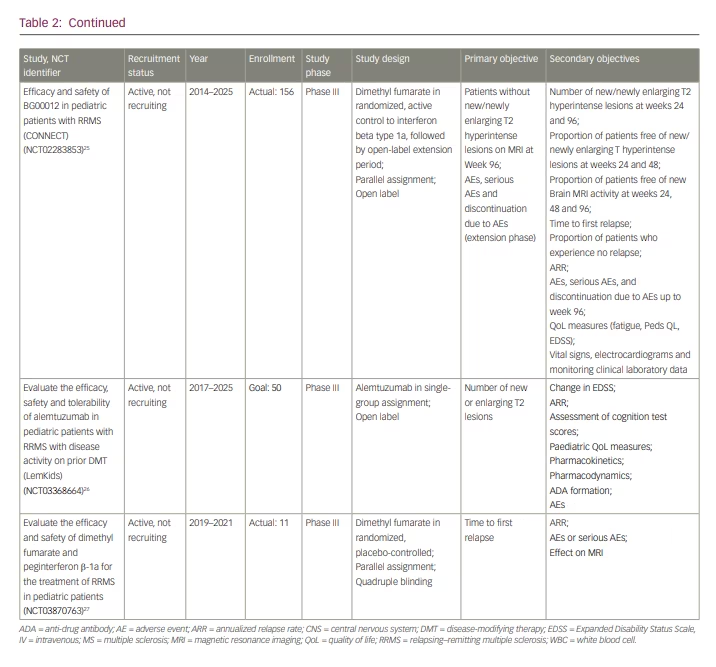
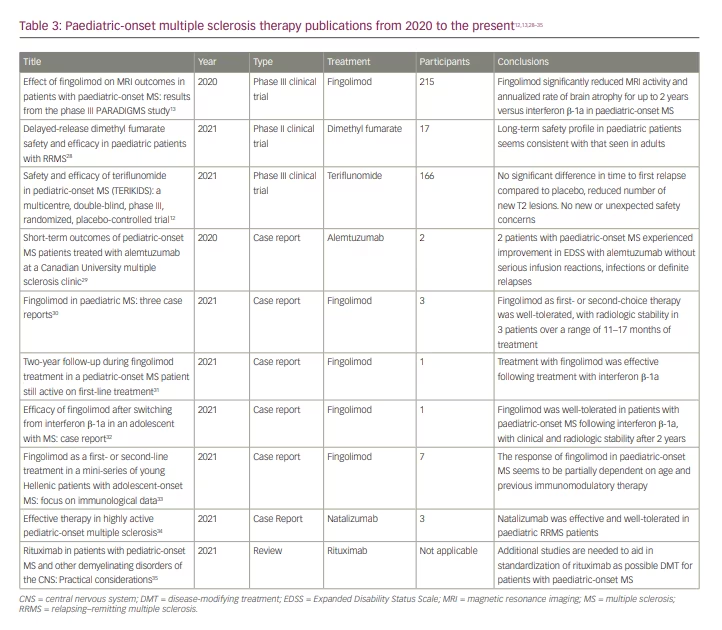
Our comprehensive literature search yielded 10 recent manuscripts on DMT use in POMS, of which there were three trial results papers and one review article (Table 3). The remainder were case reports and case series. In the first trial article, Arnold et al. reported on the predefined MRI outcomes from the trial, demonstrating that fingolimod significantly reduced MRI activity and annualized rate of brain atrophy compared to interferon β-1a (Safety and efficacy of fingolimod in pediatric patients with multiple sclerosis [PARADIGMS]; ClinicalTrials.gov identifier: NCT01892722).13,20 In the second, Alroughani et al. published the results of a 96-week extension to the phase II DMF trial for patients 13 to 17 years of age with relapsing–remitting multiple sclerosis (RRMS) (Efficacy and safety of BG00012 in pediatric patients with RRMS [CONNECT]; ClinicalTrials.gov identifier: NCT02283853, Extension study of BG00012 in pediatric subjects with relapsing remitting multiple sclerosis [FOCUS]; ClinicalTrials.gov identifier: NCT02555215).25,28,36,37 DMF is a twice daily oral activator of the nuclear factor (erythroid-derived 2)-like pathway.38 Of the patients with POMS enrolled, the authors found similar long-term safety and efficacy to that observed in the AOMS trials.28,39 Therefore, they concluded that patients with POMS might similarly benefit from treatment with DMF.28 In the paper by Chitnis et al., the results of the first randomized, double-blind, placebo-controlled, phase III trial of teriflunomide for patients with POMS were published (Efficacy, safety and pharmacokinetics of teriflunomide in pediatric patients with relapsing forms of multiple sclerosis [TERIKIDS]; ClinicalTrials.gov identifier: NCT02201108).12,19 After 96 weeks, there was no significant difference in time to first relapse compared to placebo. Of note, the transition from double-blind to open-label treatment occurred earlier and more frequently than originally anticipated, with 37 of the original 57 patients assigned to placebo completing the double-blind treatment period early due to either high MRI activity or confirmed relapses. The authors felt this decreased the study’s power and may have skewed the results against treatment efficacy. The results did demonstrate radiologic improvement in patients treated with teriflunomide, with a reduced number of new or enlarging T2 and gadolinium-enhancing T1 lesions compared to placebo. Teriflunomide was also well-tolerated with no new safety concerns as compared to adults.12 Based on these findings, teriflunomide was approved for treatment of patients with POMS ages 10 or older in the European Union in June 2021.40 The FDA, however, did not deem this data sufficient enough to justify its approval for POMS in the USA, highlighting the need for more clinical trial data.
Our search yielded one review paper, which was published in October 2021 by Ghezzi et al.35 The authors examined rituximab use in POMS and related disorders in the hopes of establishing a more standardized protocol.35 The authors began by mentioning the phase I study of ocrelizumab, which is now transitioning into phase III (Table 2). They then summarized rituximab use in AOMS and presented the available retrospective data published on rituximab use in several paediatric neuroinflammatory diseases.41 The 2013 International Pediatric MS Study Group’s position on rituximab is that it has significant potential benefit but requires further investigation to establish a standardized therapeutic protocol.42 The review authors go on to propose a practical framework for rituximab use in POMS, giving particular attention to dosing, frequency and monitoring parameters. At present, rituximab has not been formally approved for use in POMS by the FDA or the Canadian Health Products and Food Branch. Of note, it has also not been FDA-approved for AOMS. Therefore, the authors emphasized the need for additional short- and long-term data on rituximab use in POMS.35 They also raised specific concerns surrounding the increased infection risks in light of the coronavirus disease 2019 (COVID-19) pandemic.35
Our search yielded a number of new case studies. In a case series of three patients, Merô et al. explained how poor outcomes observed in almost 45% of patients with POMS taking first-generation injectables prompted the PARADIGMS and CONNECT trials to examine higher-efficacy options more closely.34 They then presented three active patients with RRMS successfully treated with natalizumab.35 A paper by Hunt and Traboulsee described two patients with POMS treated with alemtuzumab with favourable responses.29 Multiple additional case reports supported the results of the PARADIGMS trial, showing that fingolimod was well-tolerated and effective as first- or second-line therapy in POMS.30–33 This included a report of three patients by Ferilli et al. with radiologic disease stability 11–17 months after initiation of fingolimod.30 Furthermore, two separate case reports by Immovilli et al. and Amidei et al. each presented a patient with POMS who switched to fingolimod following failed interferon β-1a therapy, resulting in disease stability.31,32 Finally, a case report by Gontika et al. described seven patients, three of whom were treatment-naïve and received fingolimod.33 Their level of disease activity following treatment suggested that response to fingolimod may be at least partially dependent on age and prior DMT, with younger and treatment-naïve patients having potentially worse outcomes.33
Discussion
Based on our above comprehensive review of the literature, the most recent research provides growing support for starting patients newly diagnosed with POMS on highly effective DMTs as early as possible. The initiation of four new clinical POMS DMT trials will hopefully provide further justification for this. The results may also help facilitate payor approvals and instill providers with more confidence to prescribe newer DMTs to patients with POMS. One unanswered question is the use of newer DMTs in patients less than 10 years of age. Since this cohort represents such a small fraction of the overall MS population, post-hoc analyses often lack sufficient power and introduce additional biases. Moreover, this particularly vulnerable population presents additional ethical challenges, and a lack of standardized weight-based dosing guidelines makes prescribing newer DMTs to such patients challenging for providers. There are on-going trials looking at the pharmacodynamics of DMT use based on various weights and ages in POMS.22,26 It is also important to learn from other subspecialties in this regard, as rituximab is often the only medication considered in very young patients with POMS, with dosing regimens largely based on experience with paediatric rheumatological disorders.41
Recent legislation in both the USA and Europe has mandated a good faith effort for pharmaceutical companies to complete POMS trials.42 Clinical trials in POMS are difficult to complete for a number of reasons, including patient recruitment and the rarity of the disease. For example, only 166 patients with POMS were enrolled in the TERIKIDS trial over a 5-year period at 57 different centres across 22 countries, in comparison to its adult trial counterpart, which enrolled 1088 patients over 4 years at 127 different sites across 21 countries.11,43 As discussed in a commentary by Sormani and Waubant, the statistical significance for the primary endpoint of the TERIKIDS trials was not reached despite the same effect being observed in adult teriflunomide trials for reduction in new MRI lesions and ARR. This was likely a result of the study being underpowered due to small trial size.44 Nonetheless, the European Union deemed the results worthy enough to approve teriflunomide for use in POMS. The FDA, however, did not. Unless new strategies are applied to paediatric drug trials, underpowered studies will likely continue to hinder efforts to gain FDA approval of new DMTs to treat POMS.
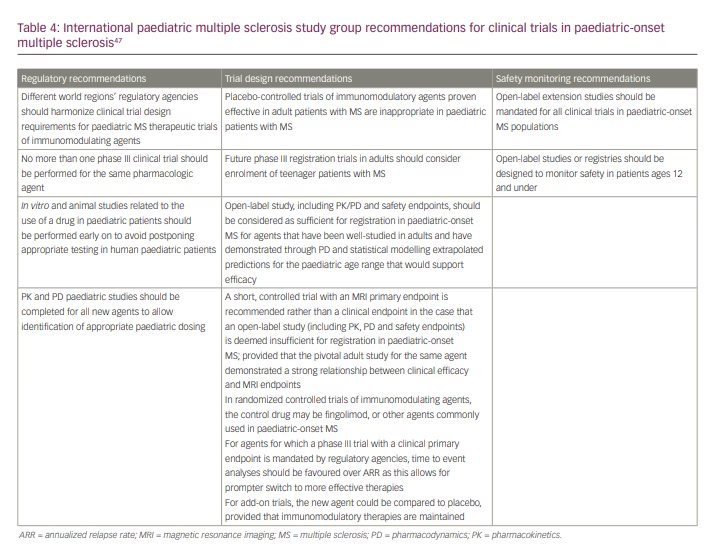
Other research barriers include the global COVID-19 pandemic, lack of consensus regarding primary outcome and quality of life measures, and ethical concerns. Additional POMS trials are necessary to evaluate the pharmacokinetics, safety and efficacy required to gain regulatory approval and to enhance medication access and long-term outcomes for patients with POMS. Unique biomarkers of POMS may help to assist in the precision of POMS clinical trials. For example, optical coherence tomography and neurofilament light chain are being evaluated as possible means to clarify the level of disease activity in POMS.45,46 A global summit was also held in 2019 to formulate 13 clear mandates to assist in designing, recruiting and completing POMS clinical trials (Table 4).47
Conclusions
The number of new highly effective DMT options for adult MS that have emerged over the past decade is impressive. When designing clinical MS trials, there is a general sense that adult trials must always be performed prior to the inclusion of paediatric patients, often due to recruitment, safety and ethical challenges.48 This can create significant delays in gaining FDA approval of adult drugs for use in the paediatric population. Fingolimod, for example, was approved for use in AOMS in 2010, but not until 2018 for POMS.49 In light of the growing evidence supporting the safety and effectiveness of adult DMTs in paediatric patients, it is worth reconsidering whether the exclusion of paediatric patients from adult research trials remains in the best interest of patients with POMS. Some adult MS trials included paediatric patients, leading to important findings. For instance, a trial of satralizumab in 2019 included adults and adolescents with neuromyelitis optica spectrum disorders, and adolescents were analysed as part of the whole cohort (Efficacy and safety study of satralizumab (SA237) as add-on therapy to treat participants with neuromyelitis optica [NMO] and NMO Spectrum Disorder [NMOSD]; ClinicalTrials.gov identifier: NCT02028884).50 This can avoid the significant delays that often occur in the completion of dedicated paediatric trials, often by several years. Priority should therefore be placed on incorporating paediatric patients into adult trials to ensure enough power is achieved to yield meaningful results. This is also important for gaining FDA approval for newer DMTs in POMS, so that these medications can become more readily accessible and available for immediate use to help prevent
long-term disability. Until such a paradigm shift occurs, the profound advances that continue to be made in the treatment of adult MS will remain under-utilized in patients with POMS, despite growing evidence that children may most benefit from such interventions.