Parkinson’s disease (PD) is characterized by prodromal and clinical stages; the clinical phase is characterized by a constellation of motor and non-motor symptoms (NMS).1 Despite the extensive discussions and publications of the clinical heterogeneity of PD,2 the precise heterogeneous pathological mechanisms, the natural history of PD and the progression pattern of many NMS remain unclear.3–7 There have also been recent publications of non-motor subtypes of PD and subtype-specific therapy strategies based on data-driven clinical studies, cluster analysis studies and animal model-based data, as explained in the included references relating to noradrenergic, cholinergic and sleep subtypes of PD, although a detailed discussion on these is beyond the scope of this article.3–8 Care for PD has traditionally focused on dopamine replacement therapies and levodopa, originally described in the 1960’s, continues to be the gold standard. There has been little advance in non-dopaminergic pharmacological therapies, especially for the management of non-motor symptoms (NMS) of PD as outlined in the Movement Disorders Society’s (MDS) evidence-based guidelines published in 2019. Many so-called novel treatment strategies of the 1990s, such as various dopamine agonists, are now used less because of fears of impulse control disorders (ICDs).9 Moreover, a dopamine replacement therapy-alone approach is inadequate for the newly defined wellness concept in PD as documented by Subramanian and colleagues.10 The need for incorporating non-dopaminergic measures, lifestyle-based activities and other wellness strategies is now considered a major enabler of good care for PD in the clinic, moving away from a dopamine replacement therapy-alone approach.10 A stepwise care approach considering the above-mentioned steps in the clinic is, therefore, a marker of good clinical practice.
In this article, we provide an overview of the proposed stepped-care strategy with supporting references for those wishing to find more detailed methodologies. For clarity, we have also included two illustrative case histories that underpin the need for the adoption of the care strategy paradigm we propose.
The unmet need and the vitals dashboard of Parkinson’s disease
Defining PD as a single disease concept is outdated and does not serve the patient and carer well. The fact that PD is not a single “disease” has been highlighted by neuropathological evidence from Jellinger, as well as clinical commentaries from Korczyn and, more recently, Titova et al.2,11,12 Yet, little attention has been paid to this concept until recently; the MDS has formed a task force aimed at unravelling the mysteries of the fact that PD is not a single disease.11–13 Non-motor subtyping of PD has been increasingly recognized and, in part, substantiated by neuropathological studies, clinical cluster analyses and data-driven cohort studies. It was first proposed in 2016 by Sauerbier et al., with subsequent publications on personalized subtype-based therapies as well as clinical personalized medicine strategies.14–16 In recent years, the role of lifestyle, physical activity and other aspects such as bone and gut health has become increasingly relevant to be considered in every clinical visit of patients with PD and is considered crucial for the wellness and culturally competent care of the patient.10 We have recently published the concept of the vitals of PD, which is a pragmatic clinical experience-led concept that consists of often-ignored symptoms, including motor and non-motor features of PD, along with domains of vision, gut and oral health, bone health and record of falls or fear of falling, comorbidities and medication side effects (Figure 1).17,18 This dashboard-based prompt for symptoms that should not be missed, has been welcomed by general practitioners as well as patient communities, and a subsequent list of tests and enablers of the dashboard has been published.18
Figure 1: The dashboard with some suggestions of potential tests that could support each domain
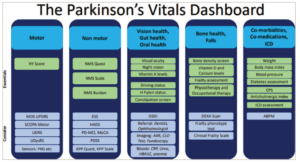
ABPM = ambulatory blood pressure monitoring; AXR = abdominal X-ray; CLO = Campylobacter-Like Organism test; CPS = Comorbidity Polypharmacy Score; CRP = C-reactive protein; DEXA = dual energy X-ray absorpiometry scan; ESS = Epworth Sleepiness Scale; HADS = Hospital Anxiety and Depression Scale; HBA1C = glycolsylated haemoglobin; HY = Hoehn and Yahr; H pylori = Helicobacter pylori; ICD = impulse control disorders; KPP = King’s Parkinson’s Pain; MCI = mild cognitive impairment; MDS = Movement Disorder Society; MoCA = Montreal Cognitive Assessment; NMS = non-motor symptoms; OSDI = Ocular Surface Disease Index; PD = Parkinson’s disease; PDSS = Parkinson’s Disease Sleep Scale; PKG = Parkinson’s KinetiGraph; UDRS = Unified Dystonia Rating Scale; UDysRS = Unified Dyskinesia Rating Scale; UPDRS = Unified Parkinson’s Disease Rating Scale.
Reproduced with permission and without amendment from Chaudhuri et al. and Qamar et al.17,18 under CC BY-NC4.0 DEED (https://creativecommons.org/licenses/by/4.0/).
In our experience, many physicians are aware and recognize that healthcare systems can be strained due to capacity and workforce issues, and clinical consultation times can be limited. The data suggest that globally, general practitioner appointments last less than 5 minutes (min) for half of the world’s population, while in the UK, the average appointment time is around 10 minutes.19 This presents a significant challenge for both patients and healthcare professionals (HCPs), as many treatable and/or significant symptoms may go unnoticed without prompt intervention from the HCP, despite patients having utilize validated tools whilst they await their assessment.
Clinical consultation in PD is typically focused on key motor symptoms and usually lasts 15–30 min. As a result, many disruptive NMS and key vital signs often go unaddressed. The use of the PD NMS questionnaire (NMSQuest), completed by patients and recommended by professional societies, has significantly improved the identification of NMS in clinical settings. NMSQuest is a patient-completed tool that is specifically validated for PD and is less time-consuming to use compared with recent tools such as MDS-NMS , which can only be completed by health professionals and is time-consuming. This improvement has led to increased awareness and better management in clinics, as demonstrated by Parkinson’s UK clinical audit of health services in the UK.20,21
A practical, simple and quick assessment of both motor and non-motor aspects of patients with PD in the clinic is now possible, as recommended by some learned societies. This assessment includes a combination of scores obtained from Hoehn and Yahr (HY) staging of PD and an NMSQuest score, which can also be graded according to severity.20 HY staging can score the motor severity of patients, ranging from mild to severe, while the NMSQuest grading ranges from no burden to extremely severe burden of NMS (more than 14 questions answered as “yes”).20
The use of these assessments and their grading can help guide symptom-specific care, which might otherwise be overlooked. Patients in the early motor stage (with a mild HY grading) may still experience a severe or extremely severe burden of NMS that requires specific attention.
Specifically, the dashboard addresses the five vitals. Domain 1 is focused on core motor symptoms and a quick validated HY stage assessment, which, despite its clinimetric limitations, remains a valid measure regularly used in clinical trials and provides the clinician with a broad overview of the motor state. Domain 2 is a record of flagged NMS and is best gathered using the globally validated patient-completed NMSQuest. The combination of HY stage and NMSQuest grading to stage patients has been reviewed and published.20 Domain 3 combines three major aspects of morbidity, which addresses vision, gut and oral health. Domain 4 addresses the important aspect of bone care in PD, which is often missed in clinical practice.17,18 Domain 5 addresses the recognition of relevant comorbidities, ensuring medications that can potentially worsen general health and cognition, such as anticholinergics and ICDs, are avoided (Figure 1). Each column represents a domain. The top heading of the columns describes the specific clinical aspects addressed by the domains and relates to the five vitals. The lower parts of the columns describe “essential” tests that could be undertaken to measure or assess the domains, such as the HY stage for the motor domain, while potential tests that could be “considered” are shown in the lower section.17,18
Illustrative case history 1
A 64-year-old patient with untreated PD presented to the clinic for management advice after experiencing stiffness in the right arm and a mild tremor in the right hand, noted by the patient and their spouse. During the clinical visit, a rapid HY assessment was conducted, revealing HY stage 1 with non-intrusive resting tremor and mild bradykinesia. Additionally, the patient completed an NMSQuest while in the waiting room, resulting in a score of 19 out of a total of 30 (Figure 2).22
Figure 2: A patient-completed non-motor symptoms questionnaire as described in the illustrative case

Each “yes” answer is considered as a point, and in this case, a total score of 19 is recorded. A score of 19 signifies an extremely severe burden of non-motor symptoms.22 The authors completed this example questionnaire as an illustrative case.
The focus of care was directed towards intrusive NMS reported in the NMSQuest, such as dysphagia, depression, anxiety and sleep dysfunction. These issues had not been recognized or addressed by the primary neurologist, as the relevant “prompts” were not completed during the clinical visit.
The use of Parkinson’s dashboard as stepped care in the clinic
The dashboard of vitals complements the motor and non-motor assessments conducted in the clinic, ensuring that the aspects of care covered within the dashboard domains are not overlooked or disregarded by HCPs.
It is worth noting that the HY scale and NMSQuest flag up signs and symptoms, which may allow for rough non-motor subtyping in the clinic, especially in the de novo or early stage of PD. Clinical examples include applying the simple CamPaIGN cohort-based strategy of asking patients to copy an intersecting pentagon (Figure 3) and check lexical fluency.23 Impairments in performing these clinical tests suggest early significant mild cognitive impairment, which, in the cholinergic subtype of PD, can progress to dementia in 8–10 years .23,24 Such patients need focused subtype-specific personalized therapies, including avoiding anticholinergics as well as the early use of cholinesterase inhibitor drugs.15,16 Similarly, the early development or declaration of pain, as well as autonomic problems and rapid eye movement sleep behavior disorder (flagged in NMSQuest), may mark the noradrenergic subtype of PD, while high idiopathic somnolence (abnormal scores on the Epworth Sleepiness Scale [ESS] or flagged in NMSQuest) may indicate the Park Sleep subtype.5,6 The dashboard would support the identification and management of such patients. A patient with the Park Sleep subtype must not be given dopamine D3 receptor-active agonists such as pramipexole or ropinirole, or allowed to drive or swim alone, for instance .5,6
Figure 3: A patient with early Parkinson’s disease showing impaired intersecting pentagon drawing suggesting the cholinergic subtype of Parkinson’s disease
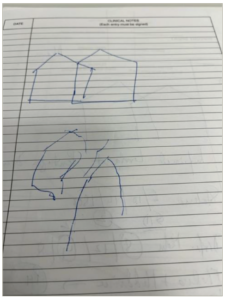
Drawn by the authors as an illustrative case.
Unawareness of the factors included in the dashboard could lead to impaired safety when performing daily living activities, as well as a potential reduction in the clinical effects of levodopa and a deterioratation in quality of life. Domain 3 of the dashboard addresses gut, oral and vision health. Gut health is particularly relevant as shown in illustrative case history 2.
Illustrative case history 2
A 56-year-old patient with an established diagnosis of PD presented to the clinic, with increasing requirements of levodopa and emergent dyskinesias. The local treating neurologist had responded to the increasing wearing off reported by the patient with frequent small doses of levodopa, which led to increasing dyskinesias. There was also a long time to ON, with each dose of levodopa particularly in the afternoon and evenings and a history of peptic ulcer disease. Recently, the patient also had two falls. In the referral centre, triage with HY stage and NMSQuest was performed, showing a high burden of gastrointestinal symptoms and the dashboard was applied. The patient was positive for Helicobacter pylori (H. pylori) infection, which was eradicated with antibiotic regimens. The patient was regularly consuming dairy products and protein either with, or after, the intake of levodopa. As per the dashboard, the patient was advised to wait at least 45 min before the consumption of dairy products after each levodopa dose. A bone scan (domain 4 of the dashboard) showed osteoporosis, which was previously undetected, and which put the patient at major risk of fractures due to their history of falls. As such, treatment for osteoporosis including vitamin D and calcium supplementation was initiated. Within 2 weeks, the patient’s levodopa requirements had fallen by 20%, dyskinesias had resolved, and time to ON had improved to 20 minutes.
As illustrative case history 2 demonstrates, patients might not realize that oral levodopa may not be working effectively because they continue to consume dairy products shortly after oral dosing. Similarly, in many cases of H. pylori infection, infection status is never checked and could be the cause of upper gastrointestinal symptoms as well as poor absorption of levodopa. Osteoporosis is common in patients with PD who are over 50 years of age, and unless specifically checked, patients may be at an increased risk of fractures if they fall, as shown in illustrated case history 2. Attention and management of these non-dopamine treatment strategies are, therefore, highly relevant to improving care for PD and substantially aided by patient-completed tools such as NMSQuest as well as the use of the dashboard. A recent international survey of the awareness of the dashboard of vitals as well as the implementation of management strategies, reported extremely limited usage of these vitals even in well-established PD clinics, with consequences such as poor absorption of levodopa, non-detection of osteoporosis and potentially dangerous daytime sleepiness (potentially leading to a risk of road traffic accidents in driving patients with PD), and sudden onset of sleep.25
The stepped-care strategy
We, therefore, propose a simple and pragmatic stepped-care strategy that can be used by HCPs in busy clinics, as shown in Figure 4.
Figure 4: A proposed stepped-care strategy for holistic and modern subtype-oriented care strategy in Parkinson’s disease
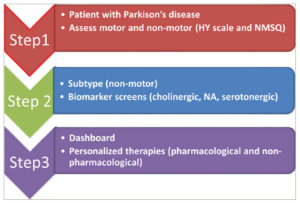
HY = Hoehn and Yahr; NA = noradrenergic; NMSQ = Non-Motor Symptoms Questionnaire. The pathway mostly applies to de novo or early PD cases.
In step 1, we propose a holistic motor and non-motor grading of PD using validated methodologies, as described, and using the time-honoured HY stage and the globally used patient-completed NMSQuest, where proxy from carers is also allowed. NMSQuest is also proposed as a necessary quality standard for the assessment of PD in clinics in many countries.21
The combined scores provide a grading as proposed by Martinez-Martin and Chaudhuri.20 The models created for validating this grading system have been developed from a cross-sectional, international study of 383 consecutive patients, where a high correlation between NMS burden (frequency × severity) and the number of NMS declared on the NMSQuest was observed. Then, based on ordinal logistic regression and percentiles of the NMS burden, cut-off values for mild, moderate and severe NMS states were validated and verified on the percentiles of another independent sample, and the cut-off values were as follows: 0 NMS = non-NMS; 1–5 NMS = mild; 6–9 NMS = moderate; 10–13 NMS = severe and >13 NMS = extremely severe.
In step 2, we propose performing clinical subtyping using simple clinical scales or tests, as also proposed in evidence-based publications.5,6,14–16 Clinical subtyping is driven by clinical examination supported by validated assessments as outlined in step 1. A completed NMSQuest would then guide the clinician to the next stage where specific subtype-based assessments can be undertaken. For instance, if a subject marks “yes” to the memory-based questions further testing for the intersecting pentagon drawing test (Figure 3), lexical fluency should be undertaken and the pathway for managing the cholinergic subtype15 should be implemented. Similarly the ESS should be used when a patient states “yes” to the relevant question on sleepiness in NMSQuest, as well as screening for historical rapid eye movement behaviour disorder, autonomic dysfunction (as declared in NMSQuest), and pain. If facilities permit in step 2, we also propose testing with relevant biomarkers or investigations as proposed by Qamar et al.18 Specifically, this may include a quantified DaTSCAN (Dopamine Transporter Scan; noting caudate: putamen binding ratio gradient), cardiac 123-I-metaiodobenzylguanidine scan, autonomic function test, formal neuropsychometry and ligand-based-specific neurotransmitter (noradrenergic, cholinergic and serotonergic) positron emission tomography scans, if available. Step 3 includes the use of the dashboard and implementation of personalized therapies based on dashboard prompt-based outcomes, as shown in illustrative case history 1 and 2, where the use of the dashboard allowed unrecognized H. pylori infection and osteoporosis to be treated, as well as dietary advice implemented. Those patients who have not had bone, gut or oral health checks must have these performed and prophylactic therapies iniated in cases where abnormal results have been observed. Those patients with impaired pentagon copying must not be given anticholinergics, and those with high somnolence scores need to be screened for sleep apnoea and should not be driving or taking dopamine D3 receptor-active dopamine agonists. Management details have been discussed in a study on PD subtype-based therapy published by Marras et al.15 The dashboard would help incorporate non-dopaminergic measures, lifestyle-based activities and other wellness strategies, which are now considered major enablers of good care for PD in the clinic, moving away from a dopamine replacement therapy-alone approach. Key wellness strategies include attention to diet, exercise, sleep, mind and body approaches and the ability to stay informed and have meaningful social engagements, all parameters that can be enabled by the dashboard. Additionally, the benefits of a subtype- and dashboard-based clinical care pathway include a potentially better clinical response to oral levodopa therapy and an opportunity to address unexplored NMS, avoiding the non-declaration of symptoms which could have a negative effect on care. The use of the dashboard prompt in the clinic could also help avoid patients suffering troublesome side effects of dopamine agonists such as sleep attacks, cognitive decline due to use of anticholinergics as well ensure awareness of bone care in vulnerable and frail patients with PD. This will lead to better overall care and wellness for the patient .26
Conclusions
PD is not a single disease and the one-size-fits-all management strategy is outdated. Neurologists and treating HCPs must become holistic in their management approach, and therapy for each patient needs to be individualized. In the setting of busy clinics and increasing capacity issues, such a management plan can be enabled using some simple paradigms covering motor symptoms and NMS along with awareness and the use of the dashboard of vitals. We have proposed a pragmatic clinical evidence-based pathway of care for people with PD that can be used in clinics in a stepwise strategy. This strategy includes not only care for the core motor and non-motor aspects of PD but also other aspects of care that drive wellness in PD, as embodied in the dashboard. There are, of course, limitations that we need to address. The proposal we make is based on a newly published dashboard for PD, and the concept of clinical subtyping needs to be validated in real-life clinical practice. Furthermore, the use of this strategy may be more complicated in the more advanced and complex stages of PD, where there may be a substantial overlap of subtypes as well as a large burden of NMS. Nevertheless, the adoption of the proposed strategy in a pragmatic clinical manner in real-life clinics is likely to result in improved quality of life and care for people with PD globally.